Iron deficiency anemia ICD 10 is the categorical classification of anemia in the International Council of Diseases 10th revision. Iron deficiency is not the sole factor for anemic conditions. Blood loss in chronic internal bleeding, hereditary diseases, and vital organ failure are some common causes of anemia. But the fact remains here, iron deficiency is the most common cause of anemia. For this liability, the International Council of Disease categorizes the iron deficiency anemia ICD 10 to highlight its significance. Even in the understanding of iron deficiency anemia ICD 10, it does not only refer to the nutritional deficiencies of iron. It is within the realm of possibility that some internal abnormalities can also trigger iron deficiency anemia in spite of taking adequate iron quantities. Erythropoietin stimulating agents serve as effective medicines in treating severe anemia.
The Backstage Study of Iron Deficiency Anemia ICD 10
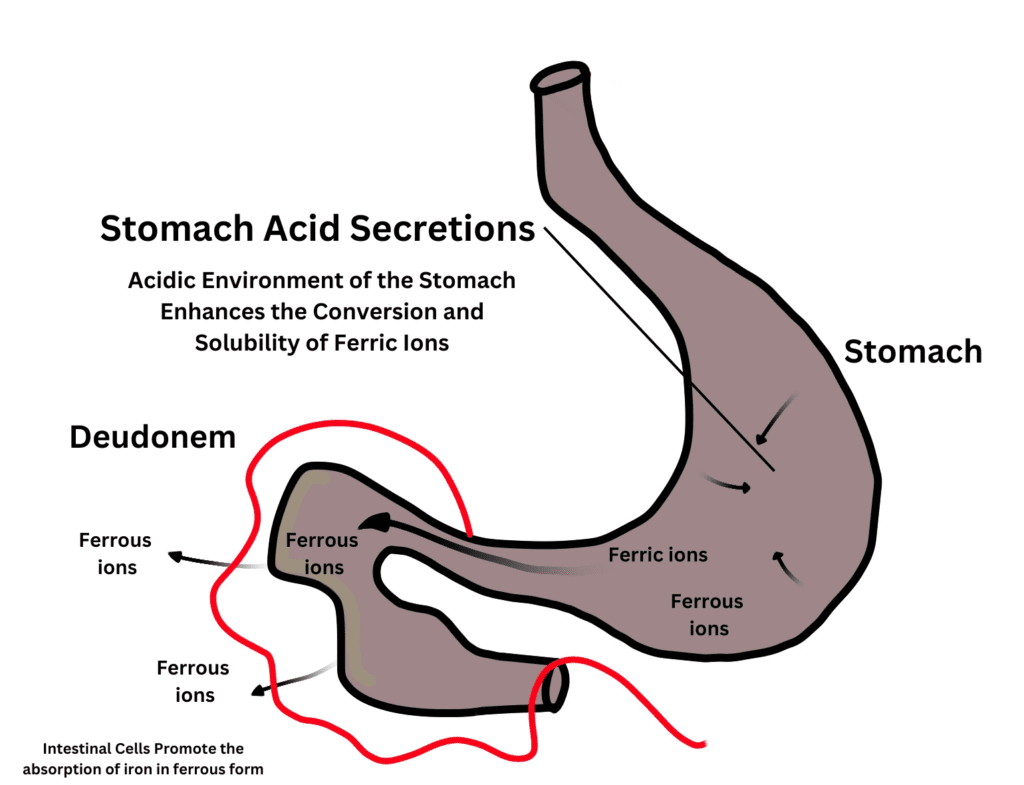
As its name prescribes, iron deficiency anemia manifests due to a lack of iron for blood cell formation. In this backstage analysis, the ferrous/ferric tension is the focal point. Red blood cells are the driving force for transporting oxygen throughout the body. Hemoglobin is the crux of red blood cells. Hemoglobin is a protein that makes 95% of red blood cells. It is the part that binds oxygen with it for its circulation to almost every cell and tissue. Hemoglobin needs iron for its structural conformity. Iron holds the central place in the structure of hemoglobin with four amino acid chains binding to it. Hemoglobin contains iron in the form of ferrous ions (Fe2+). That’s where the tension between ferrous/ferric comes into discussion.
There’s a Twist
As hemoglobin contains iron in the ferrous form, it is conceivable that the body needs iron in this form. But, the diet always does not contain ferrous ions, it can also have a ferric form of iron (Fe3+). Here’s where the distinction begins. The body converts the ferric iron into the ferrous form for its absorption. What happens if the body becomes incapable of this conversion? A person is taking a healthy amount of iron in his diet, but still, he can suffer from iron deficiency anemia ICD 10. Most of the time, this type of iron deficiency anemia ICD 10 comes under the chapter of unspecified causes. The tension between ferrous/ferric ions is in hot waters when it comes to the question of iron absorption into the bloodstream.
Ferrous Form Gains the Superiority
Our diet, both veg and non-veg, contains iron. It can either be as heme iron or non-heme iron. Heme iron is a structure that we gain from animal foods (as they contain hemoglobin). Unlikely, plants do not have active hemoglobin molecules, so they can only provide us with non-heme iron. Non-heme iron can be ferrous iron or ferric iron. Body mechanisms prefer ferrous iron as it is more liable to absorption in the intestines. Deudonem has the job of absorbing iron (mostly in the ferrous form) into the epithelial cells facing the lumen. Jejunum and ileum also have minor contributions towards iron absorption. As diet contains both ferrous and ferric iron forms, an enzyme is present in the gut for converting all available iron into the ferrous form. So, the ferrous form gains superiority over ferric iron for absorption into the body.
Causes That Spur Iron Deficiency Anemia ICD 10
Although there is one reason or the other for iron deficiency anemia ICD 10, we’ll discuss the reasons behind insufficient ferric to ferrous conversion.
Genetic Expression Vulnerabilities
The enzyme (ferric reductase) which has the root job of converting ferric ions into ferrous ones, can suffer from genetic expression vulnerabilities. Being an enzyme, it requires the linear process of transcription and translation for its synthesis. Mutations and other vulnerabilities can affect the gene that commands its synthesis. Moreover, some other proteins also have momentous roles in the absorption of iron. These proteins act as transporting or carrier proteins for iron absorption. Genetic vulnerabilities can also affect the genes that encode them.
Alternations in the Stomach Acidity
Ferric reductase works happily in an acidic environment. Stomach low pH helps the ferric reductase by providing this environment as both are close to each other. When food takes its step into the pseudonym, its contents are acidic. However, alterations in the acidic content of stomach secretion can also affect the efficiency of ferric reductase. Medications, such as Proton Pump Inhibitors (PPIs) lessen stomach acidity. Reduced stomach acidity helps treat certain conditions such as gastroesophageal reflux disease and certain stomach ulcers. But, this can also limit the absorption of many crucial nutrients, including iron.
Acidic stomach content makes the complex structures of calcium and magnesium minerals dissolve. Moreover, it facilitates ferric reductase in its job by converting the iron into the body’s favorable form, the soluble ferrous ions. Long-term use of PPIs can link to iron deficiency anemia ICD 10 or other symptoms associated with calcium and magnesium deficiencies.
Surgical Bypassing of Deudonem
Inflammatory Bowel Disease (IBD), if affects the deudonem, can cause severe inflammation in the cells of the deudonem. IBD may also result in the formation of fistulas or abscesses. Fistulas form when intestinal gaps (due to ulcers) or outgrowths of intestinal cells (due to severe inflammation) make direct physical connections with each other. Intestinal gaps can make their way to other organs or even to the other parts of the intestine itself. If such situations affect the pseudonym, triggering the narrowing of the lumen, it becomes compulsory to surgically remove it.
Before surgical bypassing, doctors try to ease the situation with anti-inflammatory drugs or foods, or lumen-dilating medications. But, if surgical removal becomes compulsory, it greatly affects the absorption of nutrients. As iron absorption mainly occurs in the pseudonym, ferric reductase activity is most active in this region, so one can assume the impact of its surgical removal.
Impacts of Some Nutrients on Iron Absorption
Certain nutrients such as vitamin C are an excellent facilitator of iron absorption. As it is discussed above, an acidic environment promotes the conversion of ferric ions into ferrous ions. The same is the study here, vitamin C (ascorbic acid) upgrades the acidic climate in the stomach and intestine. Vitamin C is also famous for its phagocytic role. Likewise, animal-derived meat has both high protein and heme-iron content. High protein content amplifies the gastric acid secretion in the stomach, which is a leg up for iron absorption. Moreover, the presence of heme-iron content in meats makes it further easier to convert the available non-heme ferric iron into the soluble ferrous form. So, it is good to take vitamin C foods (oranges, strawberries, tomatoes, etc) with the ingestion of iron-containing foods as both share a reciprocal agreement.
Consequences of Ferrous/Ferric Tension
A rise in ferric ions than ferrous ones leads to ferrous/ferric tension. It can be due to any reason, some of which are explained in the previous section. If iron deficiency anemia ICD 10 occurs due to any of the above reasons, it can be subjected to treatment. But, there can be other unspecified reasons apart from them. All these reasons point to a single consequence; a rise in ferric ions and low absorption of dietary iron.
Veg Folks Are More at The Edge of These Consequences
Plant foods have more higher proportion of ferric ions due to the unavailability of heme iron. Some plant foods, including leafy green vegetables, nuts, and seeds, have iron high in the form of ferric ions. As they avoid meat ingestion, they have low stomach acidity levels which further impact the ferric to ferrous conversion. So, it would be healthy for veg folks to consume foods that have higher levels of ferrous ions than ferric ones.
Accumulation of Ferric Ions
It is evident that the body’s rate of ferric ions absorption is much less than ferrous ions absorption. So, the presence of a high concentration of ferric ions is prone to accumulation and in turn precipitation of ferric ions. Ferric ions can form chelates and stable complexes with some foods high in dietary fibers. The formation of complexes or precipitates hinders the normal motility of food inside the small intestine. Precipitation of ferric ions can also become the cause of inflammation in intestinal walls. This intestinal wall accumulation gives rise to symptoms such as abdominal pain and cramps, bloating, bowel problems, maybe internal bleeding, etc.
Contribution to The Formation of ROS
Reactive Oxygen Species (ROS) formation is a side-by-side reaction occurring in the biology realm. The formation of ROS is not abnormal or dangerous as appropriate amounts of natural anti-oxidants are present in the body. But, if there is a tilt in this balance, it can be detrimental. In the study of ferric ions accumulation, the tilt is due to the production of excess ROS. Ferric ions initiate the reaction by reacting with already present reactive oxygen species. It results in the production of hydroxal ions (OH•). Hydroxyl ions are potent reactive species whose accumulation can damage the internal linings of organs, cells, and tissues.
A Lead to Malnutrition
Despite having a rich iron meal, the body can still lack the necessary iron for red blood cell production. Malnutrition or dietary insufficiency of iron is the most common cause of iron deficiency anemia ICD 10. In the ferrous/ferric tension, an adequate amount of iron is present in the body but owing to the body’s bias to the ferrous form, the body still lacks iron. Blood takes up soluble iron form from the intestinal lumen and converts it into ferritin. Ferritin is a reserved form of iron in the body. When the body is in need of iron, such as the production of red blood cells, ferritin serves as the necessary iron. Lack of iron in the blood and the body impairs red blood cell production, stimulating iron deficiency anemia ICD 10.
To Wrap it Up
The body demands iron (soluble form) constantly. In the sphere of iron deficiency anemia ICD 10, a patient suffers from low oxygen perfusion to the vital tissues and organs. Severe anemia also triggers chronic hypoxia due to constant hypoperfusion of oxygen. Besides focusing on consuming iron in the diet, it is also essential to check for internal problems. Ferrous/ferric tension is just a single but significant reason behind iron deficiency anemia ICD 10. Many other such reasons which seem minute to consider, but are important to focus on, impact the iron levels in the blood. So, it is advised to consult professional advice if you are experiencing the symptoms of iron deficiency anemia ICD 10, but your diet contains sufficient iron levels.


Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Yes, I’m here for help if you have any doubts in my content.